Acetic Anhydride Mp
Acetic Anhydride Mp. The mechanism of the acetylation reaction undergone by salicylic acid to afford aspirin and acetic acid as the product is illustrated below. It undergoes exothermic reaction on addition of water to form acetic acid.

 US7732116B2 Photoconductors containing N from www.google.com
US7732116B2 Photoconductors containing N from www.google.comAcetic anhydride is a strong irritant.on contact with eyes, usually with delayed action; 1 b a laboratory preparation of acetic anhydride involves the reaction of sodium acetate and acetyl chloride followed by fractional distillation. For this method, i dared to presume that considerable amounts of acetic anhydride may be formed when mixing oleum (60% oleum, for instance) with glacial acetic acid.

Wacher process (starting reagent + a ketene) and knapsack process (starting reagent = acetaldehyde) have been reported for the industrial preparation of acetic anhydride. Contact is followed by lacrimation, photophobia, conjunctivitis and corneal edema.
The resulting positive charge is relayed to the adjacent carbonyl carbon, which binds itself to the phenolic hydroxy group. Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (ch 3 co) 2 o.

It undergoes exothermic reaction on addition of water to form acetic acid. Acetic anhydride is a carboxylic acid anhydride commonly used as an acetylating agent for amines and alcohols.

Acetic anhydride, acetanhydride, acetic acid, anhydride, acetic oxide, acetyl oxide, ethanoic anhydride, acetyl ether, acetyl anhydride, acetic acid anhydride, anhydride acetique: Acetic anhydride is a carboxylic acid anhydride commonly used for the acetylation of amines and alcohols.

A mixture of acetic anhydride and acetic acid/oleum results. For this method, i dared to presume that considerable amounts of acetic anhydride may be formed when mixing oleum (60% oleum, for instance) with glacial acetic acid.
Acetic anhydride is a carboxylic acid anhydride commonly used as an acetylating agent for amines and alcohols. Acetic anhydride created by global safety management, inc.

Acetic anhydride is a reagent that is used in the manufacturing of acetyl compounds and cellulose acetates. Aniline 93.13 (184 ºc) 1.022 g/ml
Acetic anhydride can be used as a dehydrating agent for the formation of anhydrides (scheme 2). First, one oxygen atom of the acetic anhydride is protonated by the acid.
Acetic anhydride is a carboxylic acid anhydride commonly used for the acetylation of amines and alcohols. Wacher process (starting reagent + a ketene) and knapsack process (starting reagent = acetaldehyde) have been reported for the industrial preparation of acetic anhydride.

It is widely employed for the acetylation of various alcohols. Acetic anhydride is a carboxylic acid anhydride commonly used as an acetylating agent for amines and alcohols.

Acetic acid occurs in ocean water, oilfield brines, rain, and at trace concentrations in many plant and animal liquids. With alcohol forms ethyl acetate;
For this method, i dared to presume that considerable amounts of acetic anhydride may be formed when mixing oleum (60% oleum, for instance) with glacial acetic acid. 1 b a laboratory preparation of acetic anhydride involves the reaction of sodium acetate and acetyl chloride followed by fractional distillation.
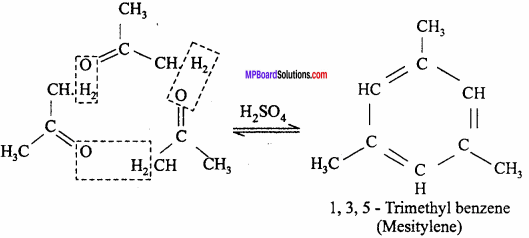
It was used in the past to treat fever and headache and was known as antifebrin by its brand name. The mechanism of the acetylation reaction undergone by salicylic acid to afford aspirin and acetic acid as the product is illustrated below.
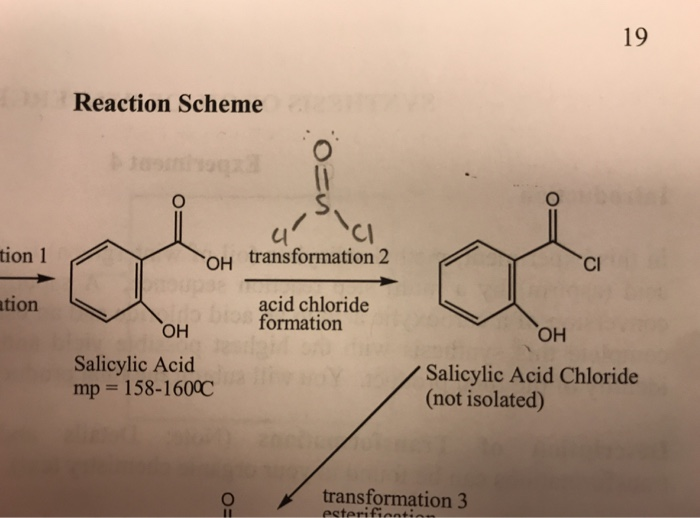
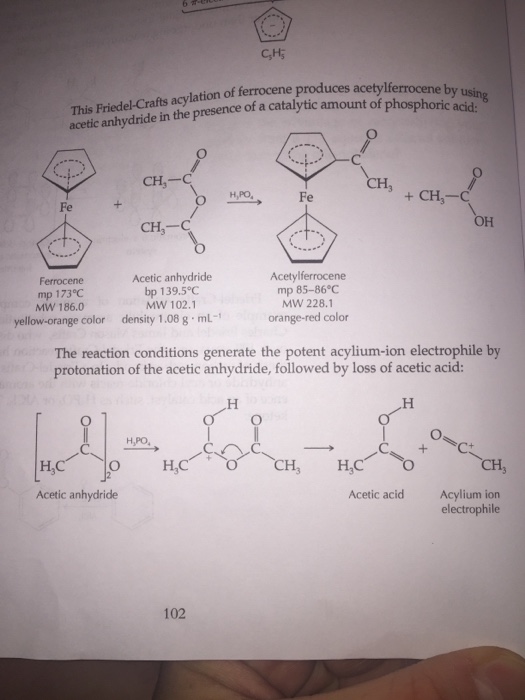
For this method, i dared to presume that considerable amounts of acetic anhydride may be formed when mixing oleum (60% oleum, for instance) with glacial acetic acid. The overall reaction for the acylation of ferrocene.
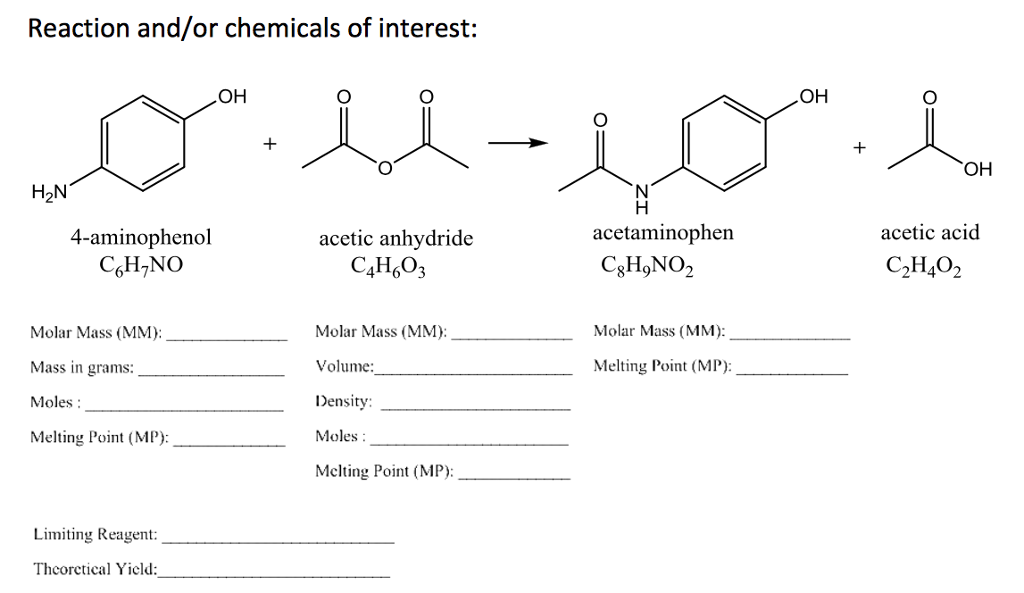
The overall reaction for the acylation of ferrocene. 1 b a laboratory preparation of acetic anhydride involves the reaction of sodium acetate and acetyl chloride followed by fractional distillation.

Acetic anhydride is a reagent that is used in the manufacturing of acetyl compounds and cellulose acetates. Smaller 3 µm particles columns available for fast uplc applications.

It undergoes exothermic reaction on addition of water to form acetic acid. Wacher process (starting reagent + a ketene) and knapsack process (starting reagent = acetaldehyde) have been reported for the industrial preparation of acetic anhydride.
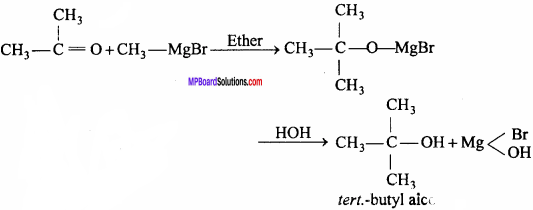
Reacts violently with boiling water, steam, strong oxidants, alcohols, amines, strong bases, and many other compounds. It is also utilized as a solvent in the examination of wool fat, glycerol, fatty and volatile oils, and resins.

The photodecomposition of acetic anhydride has been studied. Wacher process (starting reagent + a ketene) and knapsack process (starting reagent = acetaldehyde) have been reported for the industrial preparation of acetic anhydride.
Prolonged exposure may lead to pulmonary edema. Acetic anhydride is a carboxylic acid anhydride commonly used as an acetylating agent for amines and alcohols.
Mix A Big Excess Of Fuming Sulfuric Acid With Glacial Acetic Acid.This may be achieved in the absence of catalysts or with acidic or basic catalysts < 09cb3484, 25cb1418, 57ja3508, 85la1935 >. Formation of acetic acid can occur. Acetic anhydride can be used as a dehydrating agent for the formation of anhydrides (scheme 2).
Acetic Anhydride Is A Reagent That Is Used In The Manufacturing Of Acetyl Compounds And Cellulose Acetates.Smaller 3 µm particles columns available for fast uplc applications. The resulting positive charge is relayed to the adjacent carbonyl carbon, which binds itself to the phenolic hydroxy group. Aniline 93.13 (184 ºc) 1.022 g/ml
Ld 50 Orally In Rats:The mobile phase contains an acetonitrile (mecn), water, and phosphoric acid. One way to look at this molecule is to think of it as containing two Mp hartshorn, wt robinson, ag waller and gj wright.
On Dissolution In Water It Undergoes Solvolysis To Afford Acetic Acid.It undergoes exothermic reaction on addition of water to form acetic acid. 1 b a laboratory preparation of acetic anhydride involves the reaction of sodium acetate and acetyl chloride followed by fractional distillation. The effect of varying the ratio of glacial acetic acid, acetic anhydride to cotton stalk was also investigated using the response surface methods.
Wacher Process (Starting Reagent + A Ketene) And Knapsack Process (Starting Reagent = Acetaldehyde) Have Been Reported For The Industrial Preparation Of Acetic Anhydride.Application acetic anhydride may be used as a reactant to synthesize: The photodecomposition of acetic anhydride has been studied. First, one oxygen atom of the acetic anhydride is protonated by the acid.
Belum ada Komentar untuk "Acetic Anhydride Mp"
Posting Komentar